Like our environment we are all the time exposed to ionizing radiation from mainly natural sources. When a molecule loses an electron, the resulting ion undergoes subsequent reactions. Naturally, the question arises how the complex and, therefore, vulnerable biomolecular structures in living organisms can sustain and survive such damaging effects. How are organic molecules damaged through ionization and in particular how do other molecules in the close vicinity influence and modify these processes?
Researchers around Alexander Dorn of the MPI for Nuclear Physics are addressing these questions experimentally by comparing reactions of gaseous organic molecules with reactions of the same molecules with one or more attached neighbors like water. In the past, they found that ionization of a water molecule can initiate energy transfer from the water to the organic molecule ionizing and breaking it [Ren et al., Nature Physics, 2018]. In their present study, they focused at the initial and direct ionization and possible subsequent modification of the organic molecule itself.
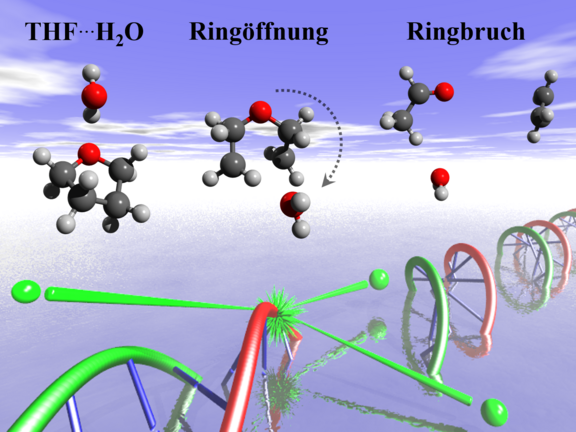
The group succeeded to identify the underlying mechanisms for biologically relevant pairs (dimers) of two tetrahydrofuran (THF) molecules or one THF and one water molecule. THF, a five-membered ring consisting of one oxygen and four carbon atoms similar to the deoxyribose rings in the DNA backbone, can serve as a model for studying radiation damage of DNA. “We bombarded a gas jet containing monomers, dimers and also larger clusters with electrons in a reaction microscope and measured the energy deposited and, simultaneously, the ion mass to see if there is fragmentation of THF”, explains Xueguang Ren, post-doctoral researcher in the group of Alexander Dorn, the experimental approach. In case of the smallest possible energy deposition, it turned out that THF ring-break occurred in dimers, while the ring structure remained stable in single molecules as well as larger clusters.
In order to gain better insight, the scientists performed theoretical calculations. “Instantaneous ionization of the organic molecule causes a sudden and strong attraction of the neighboring water molecule which consequently collides with the ring leading to the breaking of its structure”, Enliang Wang, former post-doctoral researcher in the group, summarizes the results. “The reaction pathway involves several intermediates and proton transfer as a further key step.” In other words, upon ionization of the THF in a THF∙H2O dimer, the neighboring water molecule catalyzes the ring-break reaction.
Interestingly, this damage mechanism is absent if more than one water molecule is bound, since the energy can be distributed among many molecules and this represents efficient cooling. The local aqueous environment in which biomolecules are embedded thus provides a protective shield. “Our findings underline the importance of understanding the energy transfer processes in condensed matter and that the aqueous environment in biological cells is responsible for the stability of biomolecules under irradiation”, concludes Xueguang Ren.
Original publication:
Water acting as a catalyst for electron-driven molecular break-up of tetrahydrofuran, Enliang Wang, Xueguang Ren, WoonYong Baek, Hans Rabus, Thomas Pfeifer, and Alexander Dorn, Nature Communications 04.05.2020, DOI: 10.1038/s41467-020-15958-7
Group Dorn in the Division Pfeifer