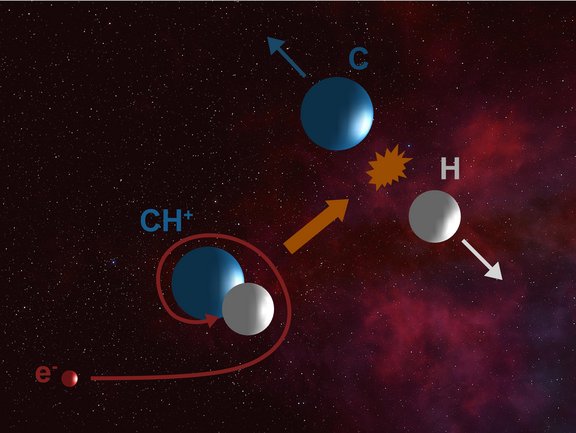
Interstellar clouds consist of extremely cold and extremely dilute gas. Although reactions between neutral atoms and molecules practically don’t take place under these conditions, nearly 300 different types of molecules are already known to exist there. The key to this surprising diversity are reactions between charged atoms and molecules (ions) and neutral reaction partners. These ion-neutral processes usually have no reaction barriers and hence they proceed at rapid rates even at very low temperatures. As early as 1941, a charged molecule was first identified in such clouds, the methylidine ion CH+.
In the 80-plus years since, the high abundance of CH+ observed in these clouds remains a big puzzle in astrophysics. One of the reasons for that are the rates of the chemical reactions forming and destroying CH+, which are currently not known for the extremely cold conditions present in the interstellar medium. Here, in contrast to the usual conditions on Earth, CH+ molecules are internally frozen, i.e., they can neither vibrate nor rotate. One of the most important reactions in the CH+ chemistry is dissociative recombination, where a positively charged CH+ ion captures a free (negatively charged) electron. The energy released in this process blows the molecule apart into its constituent atoms.
Bringing space into the laboratory
With the ultracold (cryogenic) storage ring CSR, scientists of the Max Planck Institute for Nuclear Physics have experimentally studied the dissociative recombination for internally frozen CH+ molecules. In order to stop the molecular rotation, they have stored the molecules for up to two minutes in the about 20 Kelvin cold environment of the CSR. By laser probing, they could characterise the remaining rotational quantum states and thus follow how the rotational motion slowly comes to a halt. At this point, the CH+ molecules were overlapped with a ‘cold’, i.e., moving at about the same velocity, beam of electrons to initiate the dissociative recombination process and measure its probability.
“Using combined electron and laser beams for a full understanding of a molecular reaction was quite a big challenge for the CSR team. Getting such an experiment done in a three-weeks-long experimental campaign was only possible due to the intense preparation and teamwork between technicians and scientists”, explains Oldřich Novotný, leading scientist of the campaign. And he adds: “The time and effort we have dedicated to the experiment still paid off. Because only in this way we could ensure that our results apply to internally frozen CH+ ions.
Contribution to solve the 80-year-old puzzle
“Our measurements revealed an up to a factor of 6 faster destruction of CH+ molecules by electron capture compared to assumptions in previous astrophysical models. For the first time, they provide a solid and reliable basis for future simulations of the CH+ chemistry in cold molecular clouds. They will help astrophysicists in their quest for the solution of the 80-year-old puzzle of the CH+ ion”, explains Daniel Paul, first author of the publication.
Also for other molecules, astrophysical models often rely on experimental data from ‘hot’ molecules, which are rotating or vibrating strongly. In the worst case, they even use estimated values. CSR is currently the only facility in the world where reactions of electrons with internally frozen molecules – i.e., under true space conditions – can be studied in detail. Thus, it is possible to provide the data for astrophysical models needed to interpret observational data. In particular the recently commissioned James Webb Space Telescope seems very promising to generate lots of valuable data for astronomers, which in some cases can only be fully understood with the help of complementary laboratory experiments, for example as conducted at the CSR.
Origina lpublication:
Experimental determination of the dissociative recombination rate coefficient for rotationally cold CH+ and its implications for diffuse cloud chemistry, Daniel Paul, Manfred Grieser, Florian Grussie, Robert von Hahn, Leonard W. Isberner, Ábel Kálosi, Claude Krantz, Holger Kreckel, Damian Müll, David A. Neufeld, Daniel W. Savin, Stefan Schippers, Patrick Wilhelm, Andreas Wolf, Mark G. Wolfire, and Oldřich Novotný, The Astrophysical Journal 14.11.2022, DOI: 10.3847/1538-4357/ac8e02